Amazing stuff! The magic of the carbon atom!
From the editor's summary and abstract:
"Editor’s summary
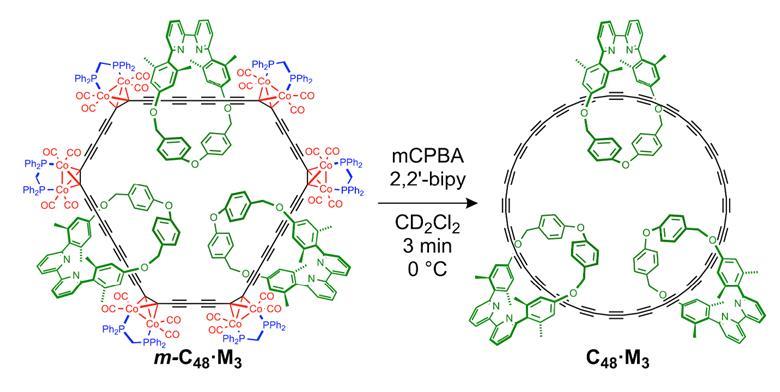
The discovery of C60 and related three-dimensional fullerenes introduced a molecular class of carbon allotropes more discrete than diamond and graphite. Recently, scanning probe microscopy has enabled the synthesis of two-dimensional molecular carbon rings, but these could only be assembled one by one on surfaces. Gao et al. now report a method to prepare macroscopic quantities of a ring comprising 48 carbons in solution phase. The key was to protect the backbone by threading it through three macrocycles during preparation of the precursor. ...
Abstract
Cyclo[N]carbons, molecular rings consisting solely of N carbon atoms, have previously been studied in the gas phase and on surfaces at cryogenic temperatures, but they are generally considered too reactive to be studied under ambient laboratory conditions.
In this study, we report the synthesis of a cyclo[48]carbon catenane, in which the C48 ring is protected by being threaded through three other macrocycles. This cyclo[48]carbon [4]catenane is stable enough for spectroscopic characterization in solution at room temperature. Its mass spectrum displays the expected molecular ions; its 13C nuclear magnetic resonance spectrum gives a single resonance for all 48 sp1 carbon atoms at 72.9 parts per million; and its Raman spectrum shows an intense peak at 1890 inverse centimeters, similar to linear polyynes."
Solution-phase stabilization of a cyclocarbon by catenane formation (no public access)
No comments:
Post a Comment