Good news! Cancer is history (soon)!
"A ready-made vaccine, already being mass produced, could offer a fast and affordable alternative to personalized jabs for stubborn pancreatic and colorectal cancers, suggests new phase 1 data. A 25-person trial found that around 70% of participants had a strong immune response to the vaccine, postponing relapse and prolonging survival. “This [vaccine] could expand treatment options for Kras-driven cancers and warrants further testing in larger trials, including exploring its potential use in lung cancers,” ... Many KRAS mutations — which drive uncontrollable cell growth in some cancers — have historically been hard to target with conventional therapies."
"A novel cancer vaccine that stimulates the immune system to target one of the most common cancer-driving mutations has shown encouraging early results in patients with pancreatic and colorectal cancer, two of the most difficult-to-treat malignancies ..."
From the abstract:
"Cellular immunity, mediated by tumor antigen-specific CD4+ and CD8+ T cells, has a critical role in the success of cancer immunotherapy by targeting intracellular driver and passenger tumor mutations.
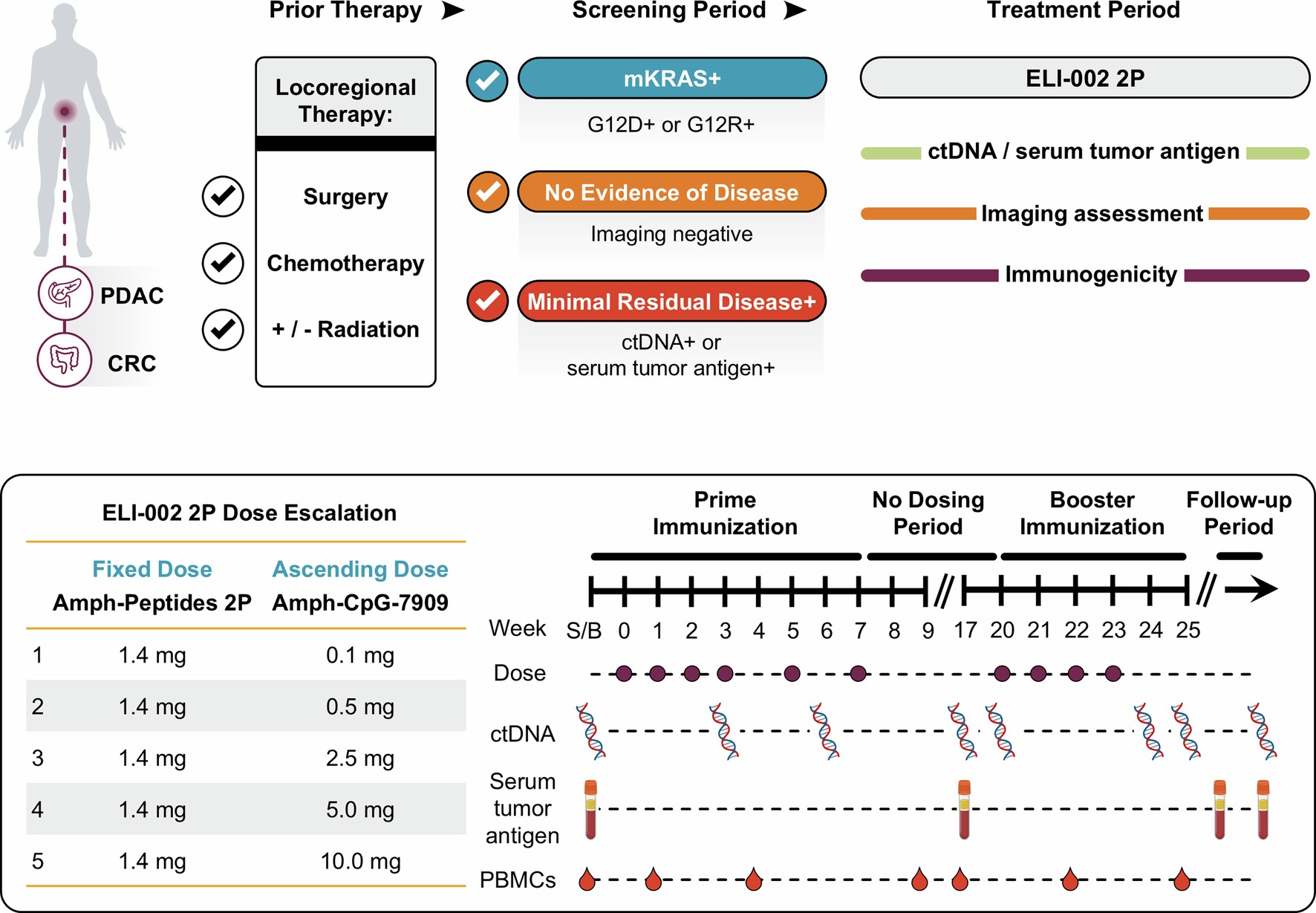
We present the final results of the phase 1 AMPLIFY-201 trial, in which patients who completed standard locoregional treatment, with minimal residual mKRAS disease (n = 25, 20 pancreatic cancer and 5 colorectal cancer), received monotherapy vaccination with lymph node-targeting ELI-002 2P, including mutant KRAS (mKRAS) amphiphile-peptide antigens (G12D, G12R) and amphiphile-adjuvant CpG-7909.
At a median follow-up of 19.7 months, efficacy correlated with mKRAS-specific T cell responses above or below a threshold 9.17-fold increase over baseline, with median radiographic relapse-free survival not reached, versus 3.02 months (hazard ratio (HR) = 0.12, P = 0.0002) and median overall survival not reached versus 15.98 months (HR = 0.23, P = 0.0099).
Seventy-one percent of evaluable patients induced both CD4+ and CD8+ subsets, with sustained immunogenicity. Following ELI-002 2P treatment, antigen spreading was observed in 67% of patients, with increased T cells reactive to personalized, tumor antigens absent from the ELI-002 2P vaccine.
Therefore, lymph node-targeting amphiphile vaccination induces persistent T cell responses targeting oncogenic driver KRAS mutations, alongside personalized, tumor antigen-specific T cells, which may correlate to clinical outcomes in pancreatic and colorectal cancer. ..."
Off-the-shelf cancer vaccine elicits strong immune response in patients with pancreatic and colorectal cancer (original news release) "Final trial results show durable T-cell responses and reduced relapse in patients with pancreatic and colorectal cancer"
Extended Data Fig. 1: Study schema.
No comments:
Post a Comment