Good news! Cancer is history (soon)!
"A Cornell-led study has revealed how a deadly form of pancreatic cancer enters the bloodstream, solving a long-standing mystery of how the disease spreads and identifying a promising target for therapy.
Pancreatic ductal adenocarcinoma is among the most lethal cancers, with fewer than 10% of patients surviving five years after diagnosis. ...
New research ... reveals that a biological receptor called ALK7 is responsible, by activating two interconnected pathways that work in tandem. One makes cancer cells more mobile through a process called epithelial-mesenchymal transition, and the other produces enzymes that physically break down the blood vessel walls. ...
The research helps resolve conflicting findings about ALK7, which some studies had linked to blocking cancer spread while others had tied to driving it. Using mouse models of pancreatic cancer and advanced organ-on-chip systems that mimic human blood vessels, the researchers showed that blocking ALK7 significantly slowed metastasis.
The organ-on-chip system, developed in Lee’s lab, simulates the tumor microenvironment and is superior to animal models for studying different stages of the cancer. Using it, the researchers studied whether ALK7 drives the initial invasion of blood vessels or the later stage, when circulating tumor cells exit the bloodstream to form new tumors in organs such as the lungs or liver. What they found was that cancer cells couldn’t enter blood vessels when ALK7 was inhibited. But when they mimicked a later stage of cancer by placing the cells inside the vessels, they spread quickly, indicating that the timing for treatment is crucial. ..."
From the abstract:
"Breaching the vascular barrier is a critical step in pancreatic ductal adenocarcinoma (PDAC) metastasis, yet the mechanisms enabling this process remain incompletely understood.
Transforming growth factor beta (TGFβ) receptors have been extensively studied in many cancer types.
However, activin receptor-like kinase 7 (ALK7), one of the TGFβ receptors, is under-investigated, and its roles in PDAC metastasis have been unclear.
This study identifies two distinct but interconnected ALK7-driven non-canonical pathways that promote PDAC dissemination.
The ALK7–β-catenin–EMT axis enhances intrinsic tumor cell motility, driving epithelial-mesenchymal transition (EMT).
In parallel, the ALK7–β-catenin–MMP axis facilitates metastatic invasion by upregulating MMP production, leading to ECM degradation and invadosome formation, which promote vascular barrier breakdown and intravasation.
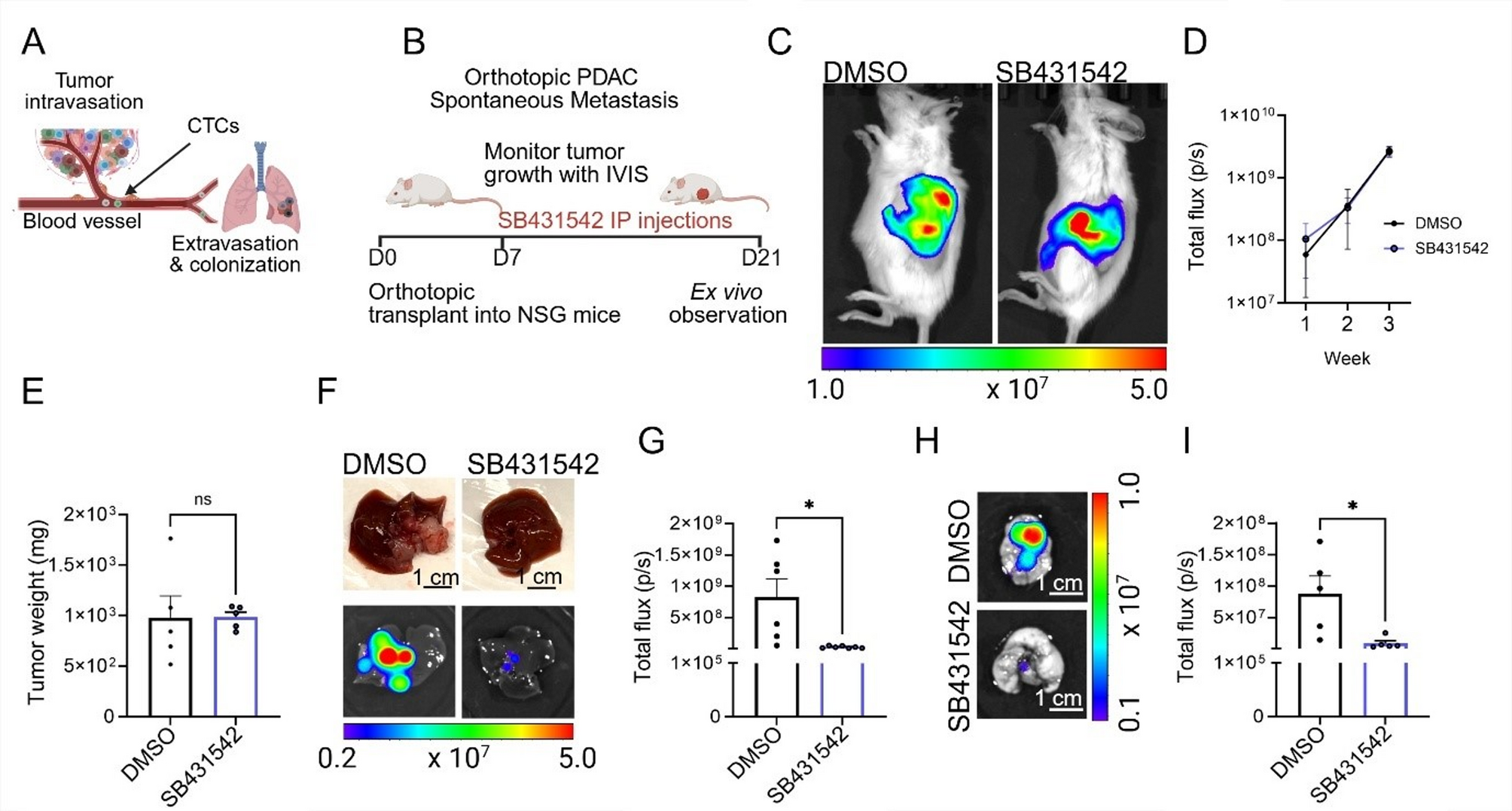
An orthotopic PDAC metastasis model reveals that both pharmacological and genetic ALK7 inhibition suppresses metastasis.
3D microfluidic vessel-on-chip platforms further demonstrate that ALK7 inhibition preserves basement membrane (BM) integrity, limiting intravasation. While MMP inhibition effectively blocks BM breakdown and intravasation, extravasation remains unaffected, highlighting distinct molecular requirements for different metastatic stages.
These findings establish ALK7 as a dual-function pro-metastatic regulator that orchestrates both tumor cell plasticity and ECM remodeling, positioning ALK7 inhibition as a promising strategy to target early metastatic dissemination in PDAC."
Fig. 1 Pharmacological ALK7 blockade inhibits spontaneous PDAC metastasis in an orthotopic PDAC model in vivo.
No comments:
Post a Comment